ANTI-INFECTIVE AGENTS (IODOPHORES, BENZALKONIUM CHLORIDE & GENTIANVIOLET)
ANTI-INFECTIVE AGENTS
INTRODUCTION
1.
Anti-infective
agents are drugs that are designed to act selectively on foreign organisms that
have invaded and infected the body.
2.
Anti-infective
range from antibiotics, antifungal, antiprotozoal, antihelmintics, antivirals
and antimycobactericals.
3.
Anti-infective
agent is an agent that is capable of acting against infection, either by
inhibiting the spread of an infectious agent or by killing the infectious agent
outright.
4.
Anti-infective
agents are the drugs utilized to exert effect on invading foreign organisms on
the body especially those which can cause infection.
5.
Its scientific
investigation started in the 1920’s after Paul Ehrlich developed synthetic
chemicals that would be effective only against the certain proteins or enzyme
system used only by infecting organisms and not by human cells.
6.
Drug resistance
remains to be the major challenge in the use of anti-infective against
infections. Emergent stains are rapidly adapting to repel the effects of anti-infectives.
7.
Anti-infective
agents in medicinal chemistry is an essential journal for all infectious
disease researchers in industry, academia and the health services.
8.
Infection is the
invasion of an organisms body tissue by disease causing agents, their
multiplication and the reaction of host tissue to the infectious agents and the
toxins they produce.
9.
Infectious
disease, also known as transmissible disease or communicable disease is illness
resulting from an infection.
10. Infections are
caused by infectious agents including viruses, viroids, prions, bacteria,
nematodes, such as parasitic roundworms and pinworms,
11. arthropods such
as ticks mites, fleus and lice , fungi such as ringworm and other
macroparasites such as tapeworm and other helminths.
12.Anti-infective agents are in reality very recent in origin prior to their discovery agents such as mercury and arsenic were used for many different ailments (most notably syphilis).Sulphonamides, which as derived from a yellow clotting die in Germany was first developed and patended in1932.
13. Historically, microbial organisms have proven to be the most prolific sources for antiinfective agents . leading to the discovery of numerous lead structures and marketed drugs .however, the number of new chemical entities that have reached clinical development and subsequently the market has substatitially decreased over the past decades, despite the emerging resistance in human pathogenic bacteria against established antibiotics.
14. During the last decades academic research has focused on the development of holistics “omics” technologies as the basis for innovative tools that allow the concise exploration of host –pathogen interactions.
15. Moreover , the detailed understanding of the molecular basis of mechanism of action and of biosynthetic process offer unprecedented options to mine and manipulate microbial secondary metabolites . The long term objectives of this , is to translate initial anti-infective hits into therapeutic leads for subsequent clinical evaluation.
The various types of infective pathogen –Using anti-infective agents in veterinary practice.
1. Antibiotics
2. Anti-helmenthics
3. Anti-ectoparasitics
4. Anti-protozoals
5. Anti-virals.
6. Anti-fungals.
General Mechanisms
The mechanisms are
a)
Inhibition the biosynthesis of bacterial cell wall
b)
Inhibition of protein synthesis.
c)
Some change the cell membrane permeability.
d) Some inhibit DNA synthesis.
ANTI-INFECTIVE agents of
clinical usefulness for systemic administration act by
inhibition of enzymatic process important in the metallic scheme of the
pathogenic organisms. The action may occure at the cell wall, preventing the
uptake of an essential metabolite or within the cell where it may block
catabolic energy supplying mechanism or anabolic protein synthesizing process.
In any case the organism loses its ability to reproduce itself.
Types of Anti-infectives
1. Amebicides
2. Aminoglycocides
3. Antihelmintics
4. Antibacterial
5. Antimalarial
6. Antituberculosis
7. Antivirals
8. Carbapenems
9. Glycopeptide antibiotics
10. Penicillins
11. Quinolones
12. Sulphonamids
13. Tetracyclins
14. Urinary anti- infectives.
Effects of Anti-infective agents
Use of anti-infectives may result to these adverse effects
Kidney damage:
Drugs like aminoglycosides have direct toxic effect on the fragile cell in the kidney and can cause conditions ranging from renal dysfunction to full –blown renal failure . Patient should be kept well –hydrate throughout drug therapy course to failure drug excretion.
Neurotoxicity:
Some anti-infectives can damage or interfere with the function of nerve tissue usually in areas where drugs tend to accumulates in high concentration e.g. aminoglycosides antibiotics collects in cranial nerve and can cause dizziness , vertigo, and the loss of hearing . chloroquine , a drug for treatment of malaria can accumulate in the retina and optic nerve and cause blindness.
Hypersensitivity:
Most agents are protein bound for transfer through the cardiovascular systems and are able to induce antibody formation in susceptible people with next explosure ,immediate or delayed allergic responses may occure.
Superinfections:
Broad spectrum anti infectives can destroy normal flora . superinfections are infections that occure when opportunistics pathogens that were kept in check by normal flora bacteria have the opportunity to invade tissue . common courses of superinfections are Proteus and Pseudomonas.
Guide to Anti-infective drug
1. Viruses: Cold,
flue, measles ,
chicken pox, polio,
yellow fever, rabies, smallpox, SARS ANTIVIRALS
2.
Bacteria: Anthrax ,
tuberculosis, gonorrhoea, plague, cholera ,typhoid, tetanus. ANTIBACTERIAL
3.
Fungi: Cryptococcosis,
aspergillosis, ringworm, candidemia, athlete’s foot. ANTIFUNGAL
4.
Protozoans:
Malaria, giardia, leishmaniasis, brain eating, amoeba, cryptosporidium. ANTIPROTOZOALS
5.
Helminths:
Pinworms ,roundworms, tapeworms, hookworms, whipworms. ANTI-HELMINTHS
6.
Algae:
Protothecosis. ANTI –ALGAL
IODOPHORE (POVIDONE –IODINE)
INTRODUCTION
1. Povidine -iodine(PVP-I) is a stable chemical complex
of polyvinyl pyrrolidone (povidone, PVP) and elemental iodine, is less toxic ,
and had been used in infected wounds and treatment of burn injuries.
2. It also known as iodopovidone
HISTORY
PVP-I was discovered in 1955
at the Industrial Toxicology Laboratories in Philadelphia by H.A.Shelanski and
M.V. Shelanski. They carried outtests in vitro to demonstrate anti-bacterial
activity, and found that the complex was less toxic in mice than tincture
iodine . Human Clinical trials showed the product to be superior to other
iodine formulations.
Following the discovery of
iodine by Bernard Courtois in 1811, it has been broadly used for the prevention
and treatment of skin infections, as well as the treatment of wounds iodine has
been recognized as an effective broad spectrum bactericide, and is also
effective against yeasts, molds, fungi, viruses, and protozoans. Drawbacks to
its use in the form of aqueous solutions include irritation at the site of
application, toxicity, and the staining of surrounding tissues. These
deficiencies were overcome by the discovery and use of PVP-I , in which the
iodine is carried in a complexed form and the concentration of free iodine is
carried in a complexed form and the concentration of free iodine is very low.
The product thus serve as an iodophore.
Structure
1. IUPAC
Name: 2-Pyrrolidinone, 1-ethynyl-homopolymer
2. Brand Name: Betadine
,Pyodine
3. Chemical formula: C6H9I2NO
4. Properties: Diluent in colour, soluble in water .
5. Structure: Povidine iodine is a chemical complex providine,
hydrogen iodide and elemental iodine.
Mechanism of action
Povidone iodine is a broad
spectrum microbicide that destroys microbial protein and DNA . It has an
excellent in vitro antimicrobial activity and is indicated for preoperative
preparation of the periocular region (lids, brow cheek) and irritating the ocular
surface. Povidone iodine is supplied as Betadine (5% sterile ophthalmic
preparation solution, Alcon)and is packaged in a single use, 1- fluid – ounce
bottle.
Povidone iodine is a combination of iodine and a water soluble polymer known as polyvinylpyrrolidone. The antimicrobial action of povidone iodine occurs after iodine disassociates from the complex. Once in free form , iodine rapidly penetrates microbial cell membranes and interacts with proteins, nucleotides and fatty acids in the cytoplasm and cytoplasmic membrane. This interaction ultimately results in rapid cell death.
Dosage
Missed dose
Apply the missed dose as soon as you remember. If is almost time for the next schedule application, then the missed dose can be skipped.
Overdose
Contact a doctor if you have used too much of povidone iodine or have accidently swallowed the medicine . Symptoms of overdose may include a metallic taste in the mouth , increases salivation, burning or pain sensation in the mouth or throat, diarrhea etc. immediate medical intervention may be needed if the overdose is sserving.
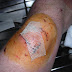
Applications of Povidone iodine
Povidone
iodine is a broad spectrum antiseptic for topical application in the treatment
and prevention of wound infection. It may
be used in first aid for minor cuts, grazes, burns, abrrations and blisters.
Povidone
iodine exhibits longer lasting antiseptic effects than tincture of iodine, due
to its slow absorption via soft tissue making it the choice for longer surgeries.
The
discovery of discovery of iodine by Burnald in 1811 it has been broadly used
for prevention and treatment of skin infection as well as treatment broad
spectrum bacteriocide and it is also effective against yeast , molds, fungi,
viruses, and protozoa.
PVP-I
can be loaded into hydrogels and gelatin or an cross-linked polyacrylamide .
these hydrogels can be used for wound dressing.
Povidone
iodine is used for the prevention and treatment of infections in the mouth.
Povidone
iodine is also used to prevent and treat the fungal infection of vagina. It may
be used in combination with bother medicines foe better results.
Povidone
iodine is also used to clean a patient’s skin before a surgical procedure is
performed.
Side effects
·
Skin irritation
·
Allergic skin reaction.
BENZALKONIUM CHLORIDE
Introduction
It is also known as
BZK,BAC,BKC or Alkyldimethyl benzyl ammonium
chloride.
Benzalkonium chloride is
quaternary ammonium compound used as biocide, a cationic surfactant, and as a
phase transfer agent.
Benzalkonium is more
commonly contained inn consumer products in its salt form, benzalkonium chloride.
This salt is used in a
great variety of international pharmaceutical products such as eye, ear, and
nasal drops, or sprays as an excipient ingredients serving as an antimicrobial preservative.
When used as ingredients
in antiseptic and disinfectant products however, it is an active antimicrobial agent.
Standard concentration are
manufactured as 50% and 80% solution , sold under trade names such as BC50,
BC80, BAC50 and BAC80.
The 50% solution is purely
aqueous while both concentrated solution requires incorporation of modifier to
prevent increase in viscosity or gel formation under low temperature.
History
It was first
introduced as a germicide in the 1910s and became more widely used in the
1940s. in the ophthalmic industry, BAC was first used in the 1940s as a mean to
preserve hard contact lens solution. Since then , BAC has been in nearly all
classes of ophthalmic solutions, from antiglucoma medicines to over the counter
artificial tear solutions.
K.C. Swan found that repeated use of benzalkonium chloride at 10-fold higher concentration of 1:5000 (0.02) or stronger can denature corneal protein and cause damage to the eye. Avoiding the use of benzalkonium chloride solutions while contact lenses are in place is discussed in the literature.
Structure
a.
IUPAC Name:
Benzyl-dimethyl-tetradecylazanium.
b.
Brand Name:
BZK,BKC,BAC
c.
Chemical formula: C6H 5CH2N(CH3)2RCI
d.
Properties:
Soluble in water and ethanol .
e.
Structure:
Benzalkonium is a quarternary ammonium compound.
Indication
When
used as an active ingredient in products like antibacterial, antiseptic or
disinfectant soaps , topical sanitizers , or cleaning agents, benzalkonium is
primarily implemented in its salt form , benzalkonium chloride ,where it may
often be the only active ingredient present and indicated for the primary
purpose of topical washing to decrease bacteria on skin.
Conversely , when
implemented as an excipient ingredient in a variety of multidose aqueous nose,
eye, or ear products, benzalkonium chloride is being used as the antimicrobial
preservative of choice to faciliate effective bactericidal and fungicidal
actions to help minimize the growth of unwanted organisms in the multidose
containers.
Pharmacodynamics
Benzalkonium chloride solutions are generally categorized as biocidal agents with relative long durations of action. Their spectrum of activity has been demonstrated against bacteria, to some viruses, fungi and protozoa , although bacterial spores are treated as being resistant,to the agent. Additionally, the agent generally shows more activity against gram- positive than gram-negative bacteria. Finally, solutions of benzalkonium chloride are bacteriostatic or bactericidal based on their concentration .Bacteriostatic agents act to prevent further growth of bacterial organisms that are present while bactericidal agents function to kill bacteria that are present. In general, the activity of the agent is not largely affected by pH, but such activity does increase substantially at higher temperatures and prolonged exposure times.
Mechanism of action
Although not entirely elucidated, the bactericidal action of benzalkonium chloride is believed to be due to the disruption of intermolecular interactions. Such disruption can cause the dissociation of cellular membrane lipid bilayers of bacteria, resulting in compromised cellular permeability control and the leakage of important cellular contents. Additionally , other important molecular complex like enzymes which control the maintenance of great range of respiratory and metabolic cellular activities, are also susceptible to such deactivation. Consequently , a variety of critical intermolecular interactions and tertiary structures in very highly specific biochemical systems that allow bacterial agents to function normally can be readily disrupted or deactivated by cationic surfactants like benzalkonium chloride.
Dosage
It is also typically used
and have liquid (1.29-1.30mg/mL), soap( 1-25mg/mL), cream( 0.005g/2g).
Application of Benzalkonium chloride
1. Benzalkonium chloride is still widely used in
eyewashes , nasal sprays hand and face washes, mouthwashes, spermicidal creams
and in various other cleaners, sanitizers, and
disinfectants.
2. Benzalkonium chloride is also used as a preservative
in nasal pharmaceuticals. There has also been concern that long term use of
benzalkonium as a preservative in nasal sprays may cause swelling of mucosa and
lead to rhinitis medicamentosa.
3. Although some studies have found no correlation
between use of benzalkonium chloride in nasal sprays and rhinitis
medicamentosa, other have found benzalkonium chloride in oxymetazoline nasal
sprays to worsen rhinitis medicamentosa in healthy volunteers after both long
term use and short term use.
4. Benzalkoinum chloride is primarily used as
preservative and antimicrobial agent, and secondarily used as a surfactant. It works by killing micro-organisms and
inhibiting their future growth, and for this reason frequently appears as an
ingredients in antibacterial handwipes, antiseptic creams anti- itch oinments.
5. The safety factor of benzalkonium chloride allows its
use in a wide range of sanitary baby wipes as well as to optimisew emolliency
and substantivity in formulations.
6. It is used as active ingredients in many consumer products.
7. Personal care product such as sanitizer, wet wipes etc.
8. It is also used in deodorants , hairproduct and cosmetics.
9. Benzalkonium chloride is used for skin antiseptics.
10. Also used on burn
and ulcer treatment.
Side effects
a. Mostly adverse effect shown by hypersensitive reactions.
b. Signs of an allergic reaction, like rash; hives;
itching ; red, swollen, blistered, or peeling skin with or without fever;
wheezing; tightness in the chest or throat ; trouble breathing , swallowing, or
talking; unusal hoarseness; or swelling of the mouth , face , lips ,tongue, or throat.
c. Swelling , redness and very bad skin irritation.
d. Effects of short term exposure , benzalkonium chloride
is corrosive to the eyes.
e. Long term effects from repeated exposure may cause dermatitis.
GENTIAN VIOLET
Introduction
1)
Gentian violet is a dye that is a mixture of violet rosanilinis with
antibacterial, antifungals and antihelminthic
properties.
2)
The name “Gentian violet” was originally used for a mixture of methyl
parasoniline dyes (methyl violet)but it is now often considered a synonym for
crystal violet.
3)
Gentian violet is a dye that is a mixture of violet rosanilinis with
antibacterial, antifungals and antihelminthic
properties.
History
Synthesis
of gentian violet was attributed French chemist Charles Lauth in 1861 under the
name of ‘Violet de Paris’ and was popularised by George Grubler, a German
pharmacist in 1880. Grubler marketed his dye only to biologist and it was not
used in textiles. In 1884 Hns Gram
noted the irreversible fixation of Gentian violet by Gram positive bacteria ,
which became the basis of the Gram stain for categorizing bacteria. Gentian
violet was first introduced as an antiseptic by Stilling in 1891, which he
marketed as pyoctanin. Stilling made boisterous claims about pyoctanins
therapeutic use and advocated it for wounds , ulcers and infections of the eye
. One physician from Vienna reported injections of pyoctanin successfully
treating two cases of Sarcoma , predating gentian violet in the treatment of
malignant melanoma and as an inhibitor of angiogenesis by over 120 years.
However , pyoctanin did not come into favor and its curative allegations were
disputed . For the next two decades , further experimentation with Gentian
violet in human subjects was abandoned.
In 1912, Churchman noted the bacteriostatic action of gentian violet against Gram positive microorganisms both in vitro and in animal studies. Based upon results from Churchman , in 1925 Hinton used Gentian violet intravenously in 12 patients with serve sepsis from Gram positive organisms, of which seven patient improved . In 1928 , a case of staphylococcal meningitis was also cured by intrathecal injections of Gentian violet . Throughout the first half of the 20th century , Gentian violet was widely adopted for use in a variety of diseases including trench mouth , thrush , impetigo, burnspinworm, cutaneous and systemic fungal infections. Claims of Gentian violet efficiency during this time period are difficult to ascertain , given that the composition of gentian violet dyes varied and the authors did not always describe the solutions used in their publications. Following discovery and mass production of sulfa drugs and penicillin in the 1940’s , Gentian violet fell out of favor with physicians and scientific research became focused on the discovery of novel classes of antibiotics.
Structure
1)
IUPAC Name: Tris[4-(dimethylamino)phenyl]methylium chloride
2)
Brand name:
Methyl violet, Brilliant violet
3)
Chemical formula: C25H30N3 CI
4) Properties: Gentian violet occurs as a solid and melting point is 215º C, Soluble in water.
Indication
for the treatment of
bacterial and fungal infections inside the mouth (thrush) and skin, also for
the prevention of transmission of Chagas’ disease ( as a blood additive).
Pharmacodynamics
Gentian violet is a
mutagen , a mitotic poison , and a clastogen. Gentian violet has been in
medicine for almost 100years: as an antiseptic for external use, as a topical
antibiotic , as a topical antifungal agent , as an antihelminthic agent by oral
administration, and more recently, as a blood additive to prevent transmission
of Chagas’ disease. It is thought to work by binding to the DNA of target
organisms and causing disruption , mutation or inhibition of DNA replication.
Mechanism of action
In aqueous solution Gentian violet (GV) dissociates into
positive (GV+) and negative ions (Cl-) that penetrate through the
wall and membrane of both gram positive and gram negative bacterial cells. The
GV+ interacts with negatively charged components of bacterial cells including
the lipopolysaccharide (on the cell wall) , the peptidoglycan and DNA. A
similar cell penetration and DNA binding process is thought to take place for
fungal cells as well . Because gentian violet is a mutagen and mitotic poison,
cell growth is consequently inhibited. A photodynamic action of gentian violet
, apparently mediated by a free – radical mechanism , has recently been
described in bacteria and the protozoan T. cruzi. Evidence also suggest that gentian violet dissipates
the bacterial (and mitochondrial) membrane potential by inducing permeability .
This is followed by respiratory by respiratory inhibition . This
anti-mitochondrial activity might explain gentian violet’s efficiency towards
both bacteria and yeast with relatively mild effects on mammalian cells.
Derivative
Gentian violet is a purple
dye derived from coal tar that is used on the skin as an antifungal and
antibacterial agent.
Dosages
The route of using gentian
violet is topical. It has variety of dosage form like tincture (1g/100mL),
solution (2g/100mL), liquid (10-20mg/1mL).
Application of Gentian violet
1)
Gentian violet is
an antiseptic dye used to treat fungal infections of the skin.(e.g. ringworm,
athlete’s foot)
2)
It also has weak
antimicrobial effects and may be used on minor cuts and scrapes to prevent infection.
3)
Also used in
bacterial, fungal, heminthic and protozoal diseases.
4)
Recent
investigational use of gentian violet has shown that it is efficacious as both
an anti-angiogenic and anti- tumor agent.
Side effects
Redness, swelling, irritation at the site of application site may occur . If any of these affects persist or worsen ,stop using this medication.














No comments:
Post a Comment